Between 18 – 21 June 2025 the Laboratory of Applied Molecular Spectroscopy (LAMS) of FORTH/ICE‑HT (Patras, Greece) joined forces with colleagues at the University of Eastern Piedmont (UPO, Italy) and Alessandria Hospital (AOU-AL, Italy) (Figure 1), to give the new SCENARIOS Raman‑Surface‑Enhanced Raman Scattering (SERS) technology its first test on biological samples – a decisive step towards routine biomonitoring of per‑ and poly‑fluoroalkyl substances (PFAS) in exposed populations.
Working side‑by‑side in UPO’s Environmental and Molecular Toxicology Laboratory, the team:
- Standardised the conditions for LabRAM HR Evolution Raman microscope (Horiba) for liquid analysis and verified wavelength and intensity calibration on site.
- Optimised the ion‑pair extraction protocol that transfers all PFAS present – from long‑chain PFOA to emerging alternatives such as ADV_M3 – into a SERS‑friendly aqueous phase.
- Demonstrated quantification down to 1 ppb in both spiked water and, crucially, human serum using replicated SERS measurements (Figures 1–2).
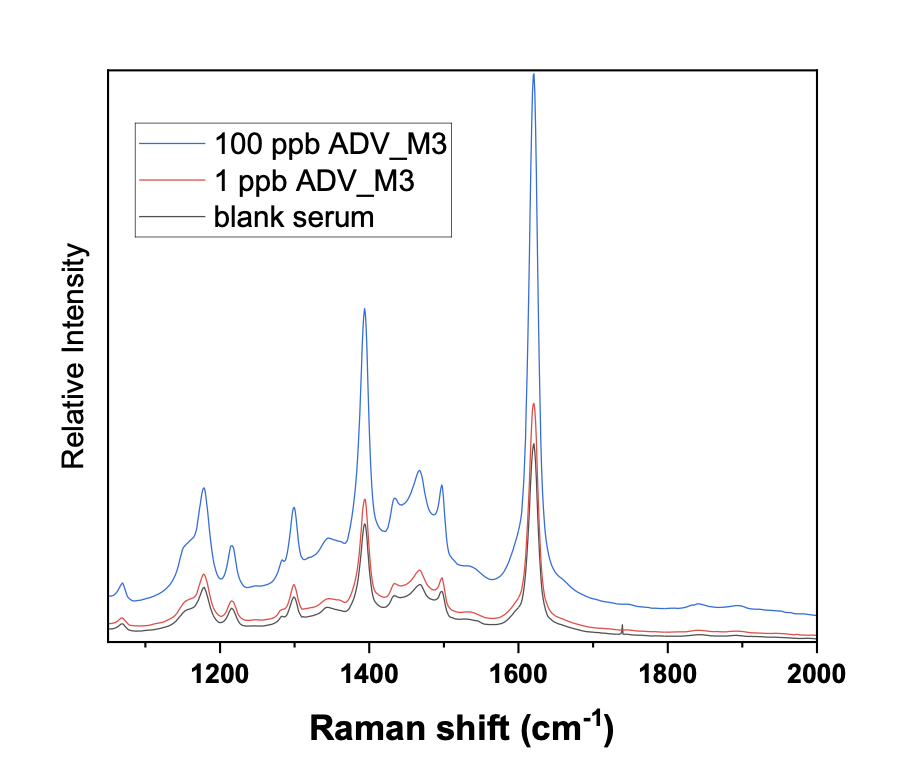
“Seeing clear, reproducible Raman fingerprints of PFAS directly in serum was the breakthrough we were aiming for,”explain Dr. George Voyiatzis and Dr. Zoi Lada (FORTH/ICE‑HT). “It proves that SERS can complement – and eventually speed up – conventional LC‑MS workflows for population‑scale exposure assessment, ”commented at the end of the hard working days Prof. Francesco Dondero (PI of the Italian team and Coordinator of the SCENARIOS project) (Figure 3).
The preliminary data were presented during the Interdisciplinary Symposium on One Health and PFAS held at the Hospital of Alessandria, sparking keen interest from clinicians eager for faster screening tools.
Why it matters
- Total PFAS rather than single analytes – the ion‑pair approach enriches all anionic PFAS simultaneously, offering a holistic exposure metric.
- Minutes instead of hours – Raman read‑out takes < 5 min per sample, compared with ≥ 120 min for targeted LC‑MS.
- Portable‑ready – the same SERS substrates can operate with field‑deployable handheld Raman devices, paving the way for on‑site blood‑testing campaigns.
The SCENARIOS consortium will next validate the method on real serum from exposed Italian cohorts and benchmark it against reference LC‑HRMS data.
