What are PFAS?
Per- and polyfluoroalkyl substances (PFAS) are man-made chemicals that are being used in almost all industry branches and that are also prominent in our daily lives. This group of chemicals comprises more than 10.000 substances. Because of their water-, fat-, and dirt-repellent properties they are used in surface coatings and can be found in numerous consumer products such as food packages, non-stick cookware, impregnations of textiles, cosmetics, firefighting foams, etc.
Why are PFAS substances of concern?
Many PFAS are extraordinary stable. They are not biodegradable and display a high thermal and chemical stability. They are called “forever chemicals” because they accumulate and persist in the environment. Worldwide, they can be found in soil, air and water, and many PFAS are frequently found in plants, animals and in human samples. Humans are exposed to PFAS via PFAS-contaminated food, drinking water, dust, air, and by the use of cosmetics. PFAS are easily resorbed into the body, but some PFAS are only poorly excreted and accumulate, e.g., in human blood. Some well-studied PFAS show numerous toxic effects, e. g. in animal studies. Based on human data, the European Food Safety Authority (EFSA) has assessed the risk to human health of four well-studied PFAS and has identified four adverse effects that are consistently associated with exposure to these four PFAS (EFSA, 2020). According to EFSA, there is an association between the exposure to these four PFAS and (i) a reduced antibody response after certain vaccinations in children (which indicates an impact of PFAS on the immune system), (ii) increased cholesterol blood serum levels (which is a risk factor for the onset of cardiovascular diseases), (iii) increased blood serum levels of the liver enzyme alanine aminotransferase (which indicates the damage of liver cells), and (iv) reduced birth weights (which may indicate putative endocrine effects of PFAS). As mentioned above, the group of PFAS comprises a large number of substances. Toxicity data are available only for a few of them, and there are many PFAS in use for which no or only few toxicity data are available. Thus, hazard characterization is hardly possible for these poor-data PFAS, and new approach methodologies (NAM) are required to generate data for the risk assessment of the large amount of poor-data PFAS.
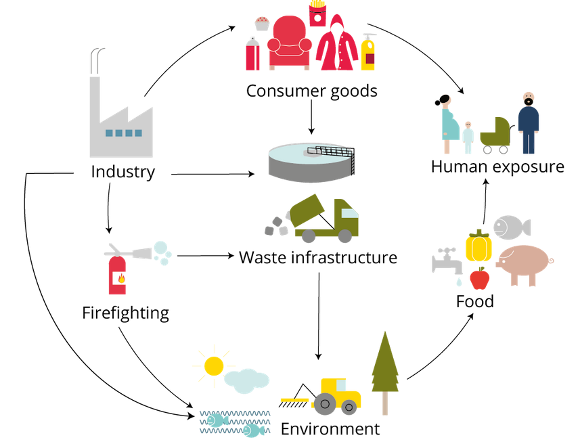
PFAS exposure routes
How can in vitro assays be used for the risk assessment of PFAS?
In vitro assays are usually based on the use of immortalized cells that have been obtained from different organs and from different species. Simple cellular models consist of only a single kind of cells whereas more complex models may be composed of several different kinds of cells and can thus pretty much mimic the features of a real-life organ. These cellular models are therefore useful tools to study toxic effects of a given test substance and the underlying molecular toxicity mechanisms at the cellular level. The toxicity data obtained with these cellular models may be extrapolated to the level of organs or even organisms to finally get a clue on potential adverse health effects in humans and animals. Moreover, in vitro assays are useful tools to compare the effects of several substances. With respect to PFAS, the effects of many poor-data PFAS can be compared with the effects of the few well-studied PFAS by means of different in vitro assays addressing various toxicological endpoints. The SCENARIOS project aims to develop an integrated approach for testing and for the assessment (IATA) for PFAS that supports the hazard characterization and risk assessment of the large number of poor-data PFAS.
Cell culture (copyright at BfR)
What kind of in vitro assays are performed within SCENARIOS?
In the SCENARIOS project, in vitro assays are being used to generate toxicity data for a number of poor-data PFAS in comparison to well-studied legacy PFAS. The work will focus on cellular models representing organs, for which adverse effects of PFAS have been described in the literature. According to the EFSA Scientific Opinion (see above), this includes cellular models for liver, immune system and endocrine system. Moreover, additional cellular models for lung, intestine and skin will be employed. By using these cellular models, numerous in vitro assays will be conducted to examine various molecular and cellular toxicity endpoints including cytotoxicity, alterations in gene expression and lipid profiles, and metabolic changes. The collective in vitrodata will provide a comprehensive picture of the potential toxicity of the tested poor-data PFAS in comparison to the well-studied legacy PFAS.
EFSA (2020) EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J-C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barreg_ard L, Ceccatelli S, Cravedi J-P, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot A-C, Van Loveren H, Vollmer G, Mackay K, Riolo F and Schwerdtle T. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 2020;18(9):6223, 391 pp. https://doi.org/10.2903/j.efsa.2020.6223